Stoichiometry AG.& ENVIRONMENTAL SCIENCES ACADEMY
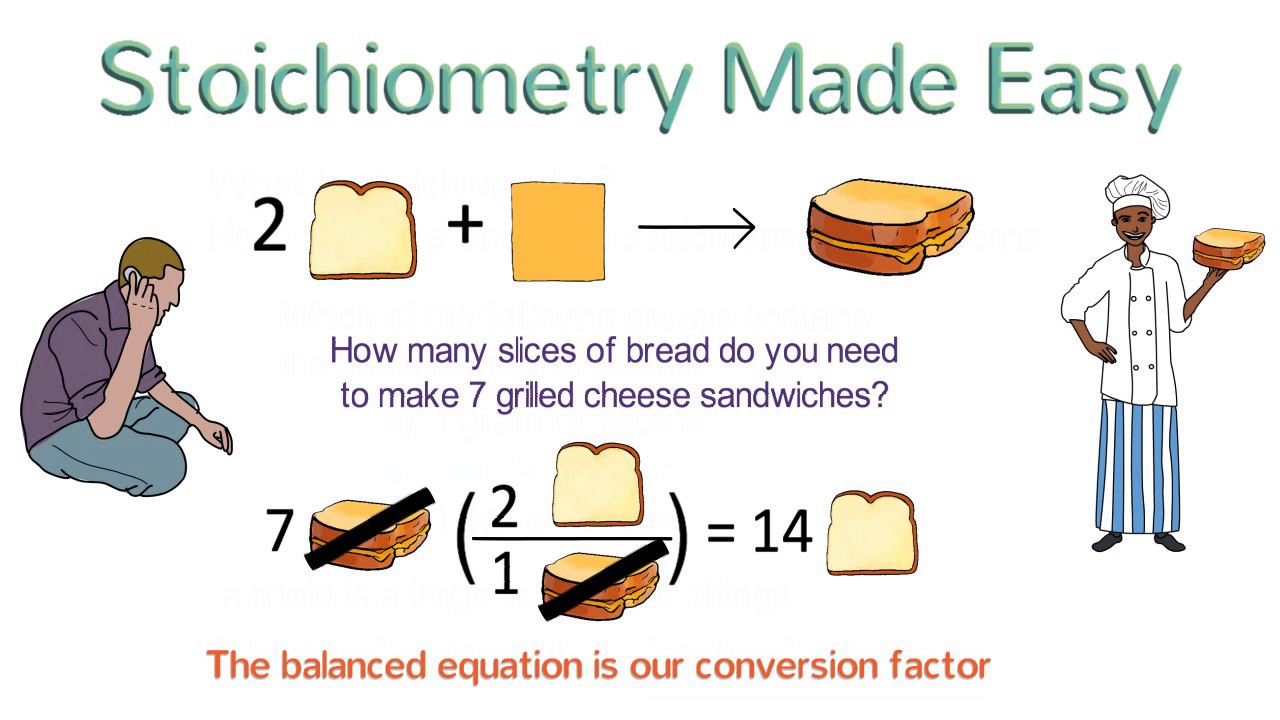
The word stoichiometry is just a fancy way of saying "the method you use to figure out how much of a chemical you can make, or how much you need, during a reaction." For example, if you‟re doing a reaction and want to make 88.5 grams of the product, you‟d do a bunch of calculations to figure out how much of each reagent you‟d need.

Stoichiometry 101
Resource Topic: Stoichiometry . The Mole, Molarity, and Density. Autograded Virtual Labs; Metals Density Problem Autograded Virtual Lab. In this activity, students use the virtual lab to identify 3 unknown metals by measuring their density and comparing their measurements to the densities of known metals. In this randomized version, each student…

Stoichiometry and Process Calculations by K.V. Narayanan
Homework Set 1: 1) 2C3H7OH + 9O2 --> 6CO2 + 8H2O a) How many moles of O2 are required to react with 58 moles of C3H7OH? b) How many moles of CO2 are required to react with 17 moles of O2? c) How many grams of CO2 would be required to react with 7.8 moles of H2O? d) How many grams of C3H7OH are needed to produce 0.45 moles of water?
Stoichiometry Limiting Reactant Science, Chemistry, Stoichiometry
Use this resource available reviewing or even assessing your students' understanding of stoichiometry and stoichiometric computation from mole till mole, mas to mole, and mass to mass. More interactively and fun than a traditional worksheet. Procure your students betrothed real answering 20 questions. Check out.

Stoichiometry Problem Set ANSWERS.pdf
$2.50 PDF Google Apps™ Easel Activity Your students can solve stoichiometry word problems and convert between moles and moles, moles and mass, and mass to mass using a chemical equation. But, what if they could have fun with a stoichiometry maze worksheet while doing so?

Stoichiometry YouTube
Q4. Given the following reaction: H2SO4 + Na2CO3 → Na2SO4 +H2O + CO2 H 2 S O 4 + N a 2 C O 3 → N a 2 S O 4 + H 2 O + C O 2. Calculate the molarity of the H2SO4 H 2 S O 4 solution if it takes 40.0 mL of H2SO4 H 2 S O 4 to neutralize 46.7 mL of a 0.364 M Na2CO3 N a 2 C O 3 solution.
4. Stoichiometry
Your students can solve stoichiometry word problems and convert between moles and moles, moles and mass, and mass to mass using a chemical equation. But, what if they could have fun with a stoichiometry practice problem hidden mystery picture worksheet while doing so? This stoichiometry calculations.

Stoichiometry Chemical reactions and stoichiometry Chemistry Khan
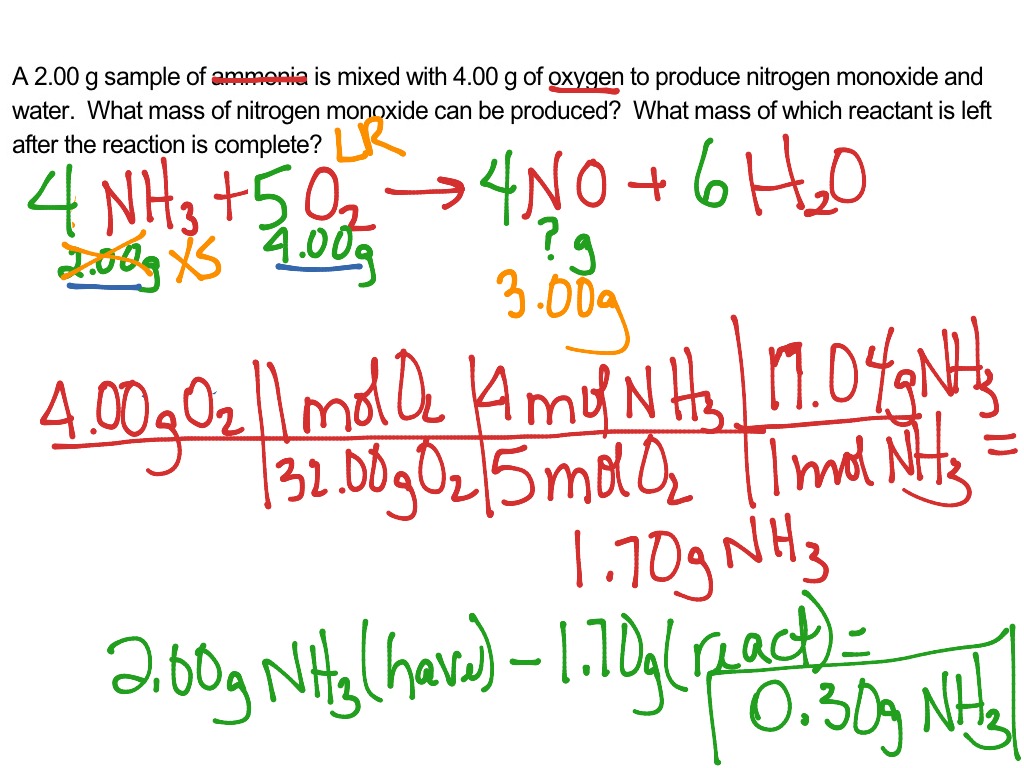
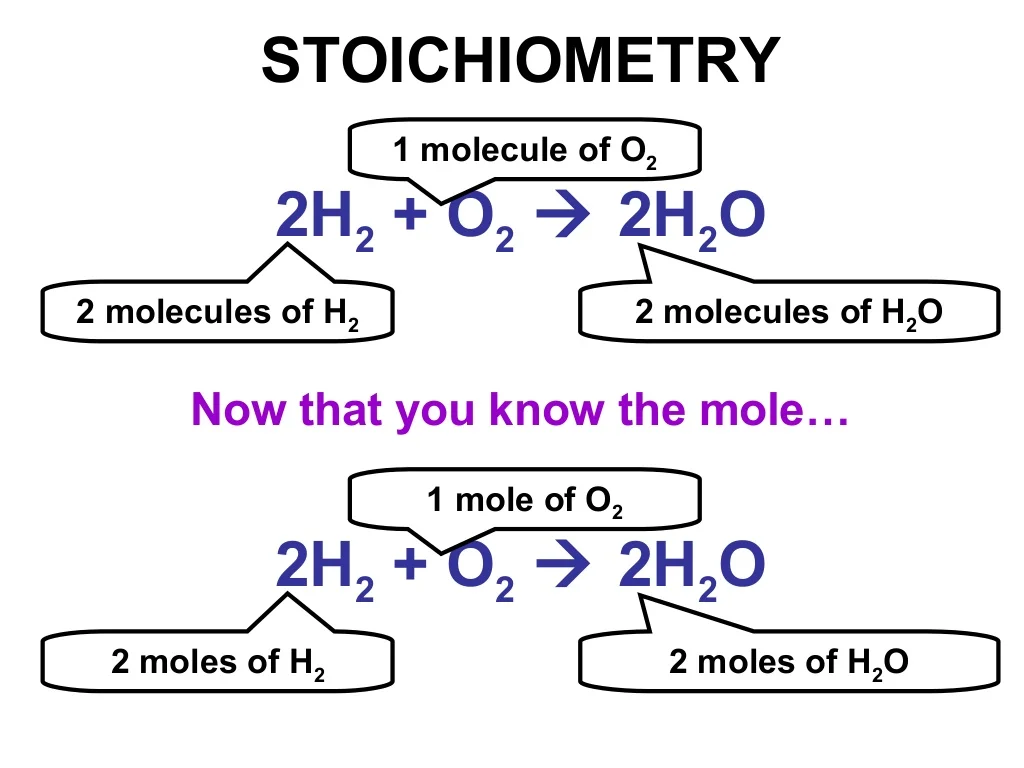
Step 1: grams of A is converted to moles by multiplying by the inverse of the molar mass. Step 2: moles of A is converted to moles of B by multiplying by the molar ratio. Step 3: moles of B is converted to grams of B by the molar mass. To illustrate this procedure, consider the combustion of glucose.
17 stoichiometry
Your students can solve stoichiometry word challenges and convert between brown and moles, moles and mass, and mass until mass using a chemical equation. But, what if handful could have fun use an stoichiometry practice issue masked mystery picture worksheet while how to? This stoichiometry calculations.

Worksheet For Basic Stoichiometry Answer
The Science Spot was developed in March 1999 by Tracy Tomm Science Teacher @ Havana Junior High, Havana, IL. Activities, lessons, & worksheets available on any page of this web site are intended for use by a single teacher in his/her classroom or to share at educational conferences. Reproduction for commercial use or profit is not permitted.

Stoichiometry Map (including moles of reaction and heat of reaction
Introduction Step by Step Stoichiometry Practice Problems | How to Pass Chemistry Melissa Maribel 341K subscribers Subscribe Subscribed 1.1M views 6 years ago What You Need to Know to Pass a Test.
Doing Stoichiometry Table method YouTube
Choose students can solve stoichiometry word problem and transform between moles and moles, moles and mass, and mass to mass using one chemical equation. But, which are they could have fun with a stoichiometry practice matter disabled mystery picture excel while doing so? This stoichiometry calculations.

Liberty Creek High School on Twitter "RT J_Fitz_SCS Investigative
Use your stoichiometry skills to identify the true murderer (s) to the police. _________________________________________________________________________________________________________________________ Suspect #1: Suave Steve Suspect #1 was arrested just outside of the mansion. He has already been imprisoned previously for nearly drowning somebody.

Stoichiometry Tutorial Pathways to Chemistry
Jake Seiler This is a review game intended for reviewing stoichiometry. Students answer questions pertaining to stoichiometry calculations and theory. If they get the question right, they are allowed to take shots to sink your ships. Each group of students gets a blank "game" board.

Stoichiometry and Solutions
No headers. Stoichiometry… what a wonderful word!It sounds so complex and so chemical.In fact, it's a fairly simple concept; stoichiometry is the relationship between the molar masses of chemical reactants and products in a given chemical reaction. In Chapter 5 we learned to balance chemical equations by inserting numerical coefficients in front of reactants or products so that there were.

maxresdefault.jpg
Your undergraduate cans solve stoichiometry term problems and convert between moles and moles, moles and mass, and mass to mas using a chemical equation. But, what if they could possess entertain with adenine stoichiometry praxis problem hidden mystery picture worksheet while making how? This stoichiometry calculations.